Fragrance oils won't mix with water because they have opposing molecular structures. Your fragrance oils contain non-polar molecules with long hydrocarbon chains that naturally repel water's polar molecules. This difference causes the oils to form separate droplets that float to the surface. While you can't achieve a stable blend naturally, you'll need specific emulsifiers like Polysorbate 20 or Solubol to create a proper mixture. Understanding the science behind this separation will help you master the art of blending these incompatible substances.
The Science Behind Oil and Water Separation

When you mix fragrance oils with water, you'll witness a fundamental principle of chemistry in action. The long hydrocarbon chains in fragrance oils naturally repel water molecules, causing them to float and separate rather than blend together.
Fragrance oils and water tell a simple story of molecular opposites, dancing briefly when mixed before inevitably drifting apart.
This separation occurs because oils and water have different molecular structures and polarities.
You'll notice that without an emulsifier, the oils will form distinct droplets that rise to the surface, creating two separate layers. Even if you shake the mixture vigorously, it'll quickly separate again.
This behavior follows the "like dissolves like" principle, where hydrophobic (water-fearing) compounds prefer to stick together rather than interact with water molecules.
That's why you'll need specific additives, such as surfactants or alcohol, to achieve a stable mixture between fragrance oils and water.
Understanding Molecular Properties of Fragrance Oils
The molecular properties of fragrance oils hold the key to understanding why they resist mixing with water. These oils consist primarily of non-polar molecules with long carbon chains that naturally repel water. Unlike some natural essential oils, synthetic fragrance oils aren't water soluble and lack the components needed for natural blending.
When you attempt to mix fragrance oils with water, you'll notice:
- Immediate separation of the oil and water layers
- Oil droplets floating on the water's surface
- Lack of uniform dispersion throughout the mixture
- Formation of distinct boundary layers
- Resistance to manual mixing efforts
To achieve a stable mixture, you'll need to use emulsifiers that can break down the oil molecules into smaller particles. These specialized additives create bridges between the oil and water molecules, allowing them to coexist in a stable solution.
Chemical Structure and Polarity Explained

Understanding polarity and chemical structure reveals why fragrance oils stubbornly resist mixing with water. When you try to combine oils and essential oils with water, you'll notice they immediately separate. This happens because of their fundamentally different molecular structures.
Water molecules are polar, meaning they've an uneven distribution of electrical charge. In contrast, fragrance oils are primarily non-polar, with their molecules having a more balanced charge distribution. Following the principle of "like dissolves like," these opposing characteristics prevent them from forming a stable mixture.
While some fragrance components contain polar groups like esters and alcohols, they're not enough to overcome the oils' overall non-polar nature.
That's why you'll need emulsifiers to create a stable blend, as they help bridge the gap between these incompatible substances.
Common Dispersants and Emulsifiers for Blending
Successfully blending fragrance oils with water requires specific dispersants and emulsifiers that act as mediators between these incompatible substances.
You'll need to understand the key elements that make these mixtures stable and effective.
Common dispersants and emulsifiers you can use include:
- Solubol, a natural emulsifier perfect for blending essential oils with water
- High-proof alcohol (190 proof minimum) mixed with oils before adding water
- Polysorbate 20, which breaks down oils into smaller, more mixable particles
- Natural dispersants that maintain long-term stability
- Professional-grade emulsifiers designed for water-based formulations
When you're working with fragrance oils, these additives aren't just helpful—they're essential for creating stable mixtures.
Without proper dispersants, your oils will separate from water, leaving you with an unstable and unusable product.
Essential Tools for Mixing Fragrance Oils With Water

Proper mixing tools and equipment make all the difference when blending fragrance oils with water.
You'll need measuring tools like pipettes or droppers for precise fragrance oil measurements, and teaspoons for your emulsifier. A glass mixing container with measurements helps you maintain accurate ratios, while spray bottles with fine mist dispensers work best for the final product.
You'll want to keep a supply of Polysorbate 20 or Solubol as your emulsifier, along with distilled water to prevent contamination.
If you're using the alcohol method, you'll need 190 proof ethanol. Don't forget to include preservatives in your toolkit to extend your mixture's shelf life.
Always have a funnel handy for transferring your blends, and keep clean, sterilized containers ready for storage.
Natural vs. Synthetic Solubility Enhancers
When mixing fragrance oils with water, you'll find both natural and synthetic solubility enhancers at your disposal.
Natural options like Solubol and Castile soap break down oils effectively while maintaining organic integrity, though they may offer less consistent results than their synthetic counterparts.
Synthetic enhancers such as Polysorbate 20 provide reliable performance in commercial applications, but you'll need to take into account their potential impact on your product's scent and texture.
Nature's Dispersing Agents
Mixing fragrance oils with water presents a fundamental challenge that nature and science have both addressed through distinct solutions.
You'll find several natural dispersing agents that can help bridge this gap effectively, making your fragrance blends more stable and consistent.
Nature offers several effective emulsifiers that you can use in your formulations:
- Grain alcohol breaks down oils into tiny particles
- Honey acts as a natural dispersing agent
- Vinegar helps stabilize oil-water mixtures
- Plant-based emulsifiers like Solubol provide gentle dispersion
- Natural waxes create stable emulsions
When you're working with natural emulsifiers, you'll notice they tend to produce milder scent profiles compared to synthetic alternatives.
While they mightn't be as potent as synthetic dispersing agents, they offer a more organic approach to achieving stable fragrance-water mixtures.
Lab-Created Emulsifying Solutions
Beyond nature's offerings, laboratory-created emulsifiers provide powerful solutions for combining fragrance oils with water.
You'll find that synthetic options like Polysorbate 20 can effectively blend oils into water-based formulations, though they may create a slightly soapy texture when used in higher concentrations.
While natural emulsifiers such as Solubol and honey work well for those seeking organic alternatives, lab-created solutions often provide more consistent results across different fragrance oil compositions.
You'll need to experiment with both types to find what works best for your specific oils.
Whether you choose natural or synthetic emulsifiers, don't forget to include preservatives in your water-based mixtures to prevent unwanted bacterial growth.
This combination guarantees your fragrance products remain stable and safe for extended periods.
Proper Dilution Ratios for Fragrance Oils
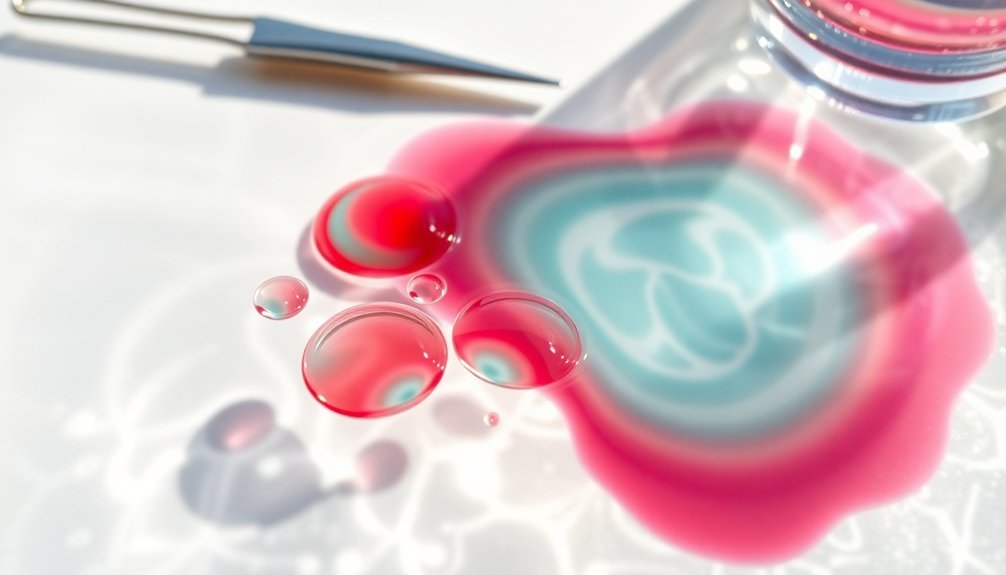
Three key principles guide the proper dilution of fragrance oils in water-based solutions.
You'll need to understand the correct ratios, use appropriate emulsifiers, and maintain consistent mixing practices to achieve ideal results.
For general applications, follow these essential dilution guidelines:
- Add 10-15 drops of fragrance oils per cup of water
- Include 1 teaspoon of emulsifier (like Solubol) for stable blending
- Use only 1-2 drops per 12-16 ounces for consumable applications
- Shake or stir regularly to maintain even distribution
- Monitor separation and re-mix as needed
Remember that fragrance oils won't naturally mix with water due to their hydrophobic properties.
That's why proper dilution ratios and emulsifiers are vital for creating stable, well-blended solutions that maintain their intended fragrance profile and effectiveness.
Safety Considerations When Mixing Oils
While proper dilution ratios set the foundation for fragrance oil blending, understanding safety protocols can protect you from potential hazards. When you're working with essential oils and fragrance oils, you'll need to follow specific safety considerations to prevent skin reactions and guarantee proper mixing.
| Safety Measure | Why It's Important | What You Should Do |
|---|---|---|
| Patch Testing | Prevents allergic reactions | Test on small skin area first |
| Proper Storage | Maintains oil quality | Use dark glass containers |
| Dilution | Prevents skin irritation | Always use a carrier oil |
| Protection | Avoids direct contact | Wear gloves while mixing |
Remember that undiluted oils can cause skin sensitivity, so you'll always want to use proper dilution ratios with a carrier oil. Store your mixtures away from heat and sunlight, and don't forget to conduct patch tests before applying any new blend to your skin.
Best Practices for Creating Stable Blends

Creating stable fragrance oil and water blends requires a thorough understanding of emulsification principles, since these oils naturally resist mixing with water.
Oil and water naturally repel each other, making proper emulsification essential for creating stable fragrance blends that last.
You'll need to use proper emulsifiers to achieve reliable results and prevent separation.
For consistently stable blends, follow these essential practices:
- Use a ratio of 10-15 drops of fragrance oils per teaspoon of emulsifier and cup of distilled water
- Select appropriate emulsifiers like Solubol, grain alcohol, or liquid Castile soap
- Choose distilled or filtered water to prevent mineral buildup
- Shake the mixture thoroughly before each use
- Create fresh blends regularly or add preservatives to extend shelf life
These guidelines will help you create properly emulsified mixtures that maintain their stability while delivering the desired fragrance effect.
Remember that without proper emulsification, your fragrance oils won't disperse effectively in water.
Troubleshooting Common Mixing Problems
Despite following proper mixing procedures, you may encounter several common issues when blending fragrance oils with water.
If you notice your oils mix floating on top, you're likely not using enough emulsifier. Try increasing the amount gradually until you achieve proper dispersion.
When your mixture becomes cloudy or separates quickly, you'll need to shake it more vigorously and possibly add more solubilizer.
Watch out for color changes or scent alterations, which often indicate a chemical reaction with high water concentrations. To prevent this, always dilute your essential oils with an emulsifier first before adding them to water.
If your blend develops an unusual smell or appearance over time, you may need to adjust your preservation system or consider using a different type of emulsifier for better stability.
Storage and Shelf Life of Oil-Water Mixtures
When storing your oil-water fragrance mixtures, you'll want to choose glass containers over plastic ones to better protect the blend from degradation and extend its usability.
You'll need a reliable preservative system since water-based solutions are particularly prone to bacterial growth and contamination.
Dark amber or cobalt glass bottles stored in cool conditions will give you the best results for maintaining the quality and safety of your oil-water fragrance blends.
Proper Container Selection
The success of your fragrance oil-water mixtures largely depends on proper container selection and storage methods.
When storing these mixtures, you'll want to choose glass containers to prevent unwanted chemical reactions that could compromise your blend's quality. Glass containers provide the best protection and help maintain the mixture's integrity over time.
Here's what you need to take into account for ideal storage:
- Select dark glass containers to protect against light exposure
- Verify containers have airtight seals to prevent evaporation
- Label each container with creation dates to track shelf life
- Store in cool, dark places to maintain scent quality
- Regularly check for signs of separation or changes in appearance
Preservative Systems Matter
Building on proper container selection, you'll need an effective preservative system to protect your fragrance oil-water mixtures from contamination and degradation. When creating cleaning products or blending water for a rejuvenating fragrance spray, preservative systems become essential for maintaining product safety and longevity.
| Preservative Type | Function | Best For |
|---|---|---|
| Ethanol | Antibacterial & Solvent | Quick-use sprays |
| Natural Systems | Gentle preservation | Sensitive formulas |
| Chemical Blends | Long-term stability | Commercial products |
You'll find that oil-water mixtures require new blending every few weeks, even with preservatives in place. To maximize shelf life, store your products in cool, dark places and always use appropriate preservative concentrations. This approach helps prevent bacterial growth while maintaining your mixture's effectiveness and safety over time.
Professional Techniques for Oil-Water Emulsification
Professional oil-water emulsification requires proper understanding of solubility principles and precise measurements to achieve stable results.
When you're blending oils with water, you'll need to use specialized emulsifiers to create a stable mixture that won't separate.
- Use a ratio of 10-15 drops of fragrance oil to 1 teaspoon of emulsifier and 1 cup of distilled water
- Select appropriate emulsifiers like Solubol, Polysorbate 20, or grain alcohol
- Maintain consistent agitation through shaking or automatic stirring
- Document your process and ratios for each oil-water mixture
- Test different emulsifier combinations for challenging oils
For oil-water mixtures that are particularly difficult to stabilize, you'll want to pay extra attention to the emulsification process.
Remember that fragrance oils are naturally hydrophobic, so you're creating a temporary suspension rather than a true solution.
Regular shaking before use helps maintain the blend's effectiveness.
Frequently Asked Questions
Can Fragrance Oil Be Mixed With Water?
You can't directly mix fragrance oils with water since they're hydrophobic. You'll need to use an emulsifier like Solubol, alcohol, or Castile soap to properly blend them together and prevent separation.
How Do You Dissolve Fragrance Oil in Water?
You'll need to mix your fragrance oil with an emulsifier like Solubol, Castile soap, or high-proof alcohol first. Then blend this mixture into water, following a 10-15 drops per cup ratio. Shake well before use.
What Happens When You Mix Essential Oils With Water?
When you mix essential oils with water, they'll float and separate since they're hydrophobic. You'll need an emulsifier like Solubol to properly blend them. Without it, you'll get uneven distribution and potential skin irritation.
How to Make Fragrance Water Soluble?
You'll need to mix your fragrance oils with an emulsifier like Solubol, grain alcohol, or liquid Castile soap first. Combine 10-15 drops oil with 1 teaspoon emulsifier, then add water and shake well.
In Summary
You'll find that successfully mixing fragrance oils with water requires understanding basic chemistry and using the right tools. By selecting appropriate emulsifiers, following proper mixing techniques, and maintaining stable storage conditions, you can create lasting oil-water blends. Don't forget to test small batches first and adjust your formulations as needed. With practice, you'll master the art of fragrance oil emulsification.





Leave a Reply